electron configuration of ag|3.1: Electron Configurations : Tagatay Click on above elements (in Periodic table) to see their information or Visit .
Manila Zoo has an online ticket booking system that enables you to purchase your entrance ticket online as well as book your visit to the park. Here are the steps. Step 1: Visit the Official site of Manila Zoo and Go to Tickets. Its official page is www.manilazoo.ph. On the homepage, go to Tickets located at the upper right side of .
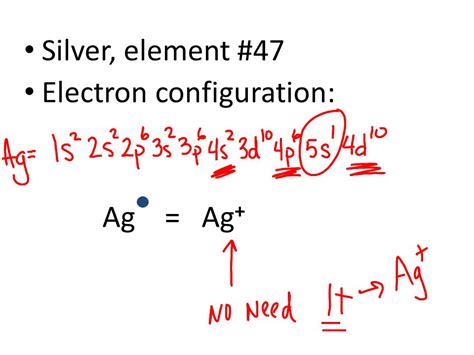
electron configuration of ag,2.4 Electron Configurations - Chemistry LibreTextsWhat is the electron configuration of Ag? | SocraticElectron Configuration for Silver (Ag and Ag+ ion) - ValenceelectronsHow to Write the Electron Configuration for Ag and Ag+ Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit .3.1: Electron Configurations To write the configuration for the Silver and the Silver ion, first we need to write the electron configuration for just Silver (Ag). We first need to find the number of electrons for the Ag.electron configuration of ag 3.1: Electron Configurations Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .

Isotopes. ⬆ ⬇. Other info. ⬆. Get the facts about element Silver (Ag) [47] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state .Electronic configuration of the Silver atom. Valence electrons. Orbital diagram.
electron configuration of agSilver is a transition metal with symbol Ag and atomic number 47. Its electron configuration is [Kr] 4d 10 5s 1, with one valence electron and one valency electron.
Element Silver (Ag), Group 11, Atomic Number 47, d-block, Mass 107.868. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .The electron configuration of Ag can be determined by using an orbital diagram. The orbital diagram for Ag can be represented by filling in the energy levels and sublevels with electrons. The first two electrons occupy the 1s orbital, followed by the 2s and 2p orbitals, which can hold a maximum of 8 electrons. .
Silver (Ag) element properties, information, facts, uses and Periodic Table trends. Complete information about the Silver element - Atomic Number 47, atomic mass [107.8682], melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, . Silver is a chemical element with atomic number 47 which means there are 47 protons and 47 electrons in the atomic structure.The chemical symbol for Silver is Ag. Electron Configuration and Oxidation States of Silver. Electron configuration of Silver is [Kr] 4d10 5s1. Possible oxidation states are +1. Electron Configuration
3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration. Na has the same electron configuration as Ne with the addition of 3s 1. Na's noble gas configuration is [Ne]3s 1. Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n – 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum . The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in .
Comprehensive information for the element Silver - Ag is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 1; Electrons per Energy Level: 2,8,18,18,1 Shell Model .
An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled . Electron Configuration For Silver: Silver is a chemical element that has a chemical symbol, Ag.The atomic number of silver is 47. It is a white, soft, lustrous transition metal. Silver exhibits the highest .Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. . Silver (Ag) 47: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1 4d 10: Cadmium (Cd) 48: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10: Indium (In) 49: 1s 2 2s 2 2p 6 . Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, .
Check me out: http://www.chemistnate.comSilver is a chemical element of the periodic table with chemical symbol Ag and atomic number 47 with an atomic weight of 107.868 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 1: Electrons per shell: 2, 8, 18, 18, 1: Valence electrons : 1: Valency electrons : 1: Where is your Periodic Table? For "silver", Z=47. It is thus 11 protons removed from the last Noble Gas, which is "krypton", Z=36. And thus the electronic configuration of silver metal is: [Kr]4d^(10)5s^1. The Ag(+I) oxidation state can be rationalized on this basis.
Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).
electron configuration of ag|3.1: Electron Configurations
PH0 · What is the electron configuration of Ag?
PH1 · Silver (Ag) [47] — Chemical Element — Periodic Table
PH2 · Silver (Ag)
PH3 · Silver
PH4 · How to Write the Electron Configuration for Ag and Ag+
PH5 · Electron configuration for Silver (element 47). Orbital diagram
PH6 · Electron Configuration for Silver (Ag and Ag+ ion)
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · 3.1: Electron Configurations
PH9 · 2.6: Electron Configurations